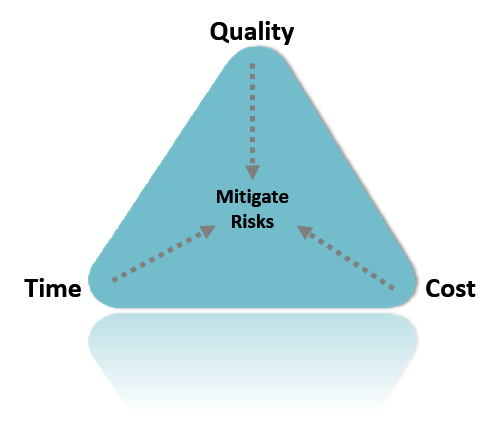
We secure Quality-Time-Cost and mitigate risks
We prioritize quality, time, and cost and take proactive steps to mitigate potential risks. By implementing rigorous quality control measures and efficient project management techniques, we ensure that your project is completed on time, within budget, and to the highest standards of quality. Additionally, we identify and assess potential risks at each stage of the project and take necessary steps to minimize their impact on the overall outcome.

Our operation process
The following diagram shows the general development phases of our medical devices CDMO solutions project, which our Taiwan headquarter mainly handles the project management and product design and our production facility at Kunshan, the PRC handles the mass production.
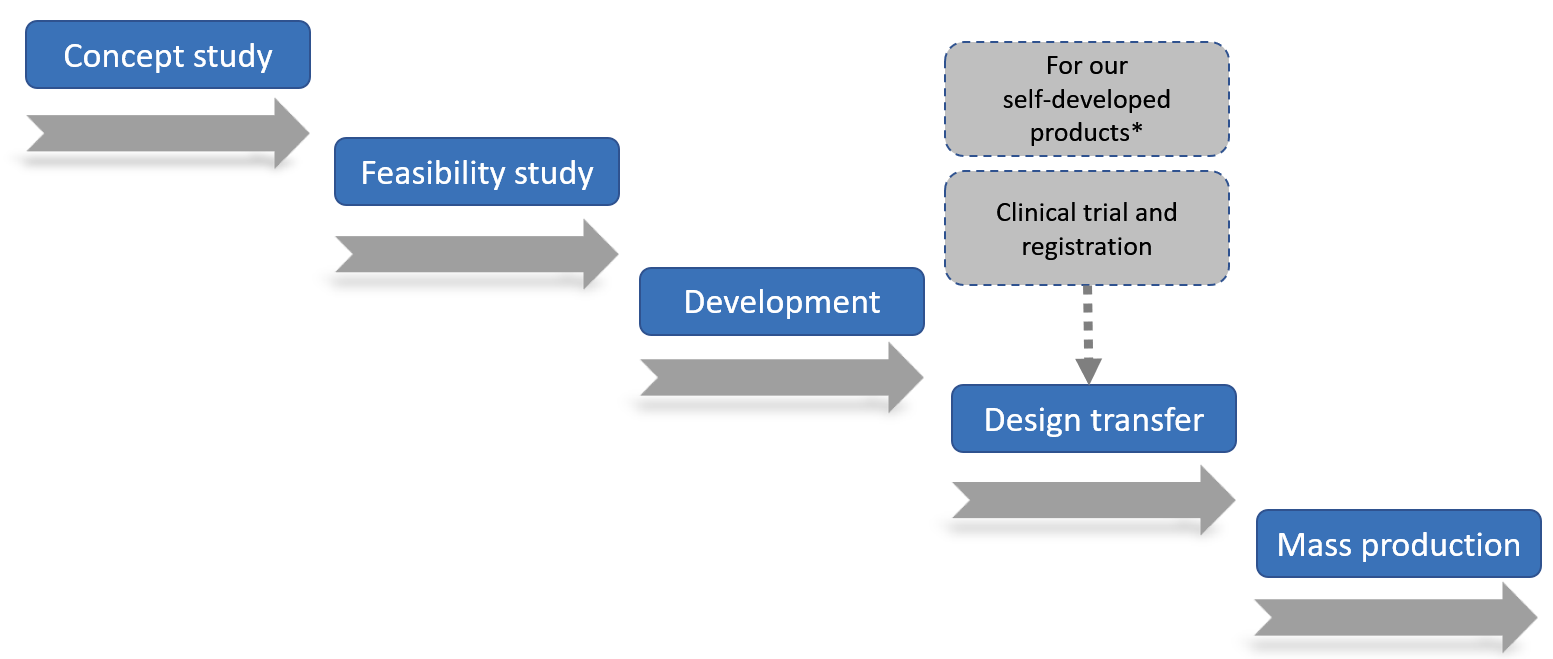
* For CDMO projects, the clinical trial and registration is handled by our CDMO customers with us providing DHF and other technical support
When our customer approaches us for CDMO solutions in the design, development and production of medical devices such as component development of new products or enhancement of existing products, we would form a cross-department project team to manage the project. Our operation process typically consists of five phases, namely,
• concept study
• feasibility
• development
• design transfer/clinical trial and registration (for our self-developed products)
• mass production
